Titration: structure and execution

With the aid of titration, the unknown concentration of a quantity of substance can be determined by titrating another substance in it. Various methods can be considered for this procedure. We explain here for you which reaction types are possible, what to look out for in the titration setup and how to determine the end point correctly.
Titration – What is it?
A titration is an experimental procedure in which the concentration of a substance can be determined. This can be useful for a variety of reasons – for example, the molar concentration of acids and bases is not always indicated on the packaging. In addition, various industries use titration for, for example:
- the manufacture of drugs
- manufacturing of foods
- the monitoring of water, soil and environment
For classical acid-base titration, a measured solution or reagent solution is used for which the substance concentration is known. This is dripped into the sample solution with the unknown concentration and a color indicator until there is a color change. This point is called the equivalence point: The substance concentrations of acid and base are equal there. Based on the consumption of the measuring solution, it is possible to calculate which concentration of the substance is present in the sample solution.
Depending on what kind of acid-base pair is involved, the equivalence point can be at different places. For the combination of strong acid and strong base, for example, it corresponds to the neutral point, so the indicator must allow a color change at a pH of 7.
Titration methods
Several methods can be used for the titration analysis procedure, which differ from one another in terms of the way they are carried out and also in terms of their structure. In principle, a distinction can be made between direct, indirect and automatic titration.
Direct titration
Direct titration is the term used when the dimensional solution and the sample solution are directly reacted with each other during the procedure. This means that either the sample solution is dripped directly into the measured solution or a previously measured amount of the measured solution is titrated with the sample solution. In the second case, it is also referred to as inverse titration.
Indirect titration
.
In indirect titration, an intermediate step is inserted because the measured solution must first be converted in a chemical reaction. In the following titration, three methods can again be distinguished:
- Indirect titration in the narrower sense: First, the sample solution is converted in a chemical reaction, whereby the resulting substance has been precisely determined beforehand. This is subsequently determined with the aid of titration.
- Back titration: Back titration, on the other hand, is based on the principle that first the entire sample solution is completely reacted with a measured solution, where this has a known volume. The remaining, unused portion of the reagent solution can subsequently be titrated and determined.
- Substitution titration: Here, the substance to be determined first releases another substance (it consequently “substitutes” it), which in turn can be back-titrated.
Automatic titration
Third in the group is the automatic titration. In this method, the pH of the solution is determined with a PC, so that the titration liquid is dribbled in completely automatically. This eliminates human error, for example. The system not only handles the evaluation, but also enables direct further processing of the measurement results. This also allows the concentration value to be calculated without an intermediate step.
Principle of the titration setup
Although there are diverse methods and reaction types, the principle of the titration setup is best explained using the classic acid-base titration. This is most easily understood using hydrochloric acid (HCl) and sodium hydroxide (NaOH) as a strong acid-base pair. Here, several basic principles are assumed:
- Measurement and volume definition: To find out the unknown substance concentration, the titrant must be dissolved in a defined volume of solvent (mostly water). This can be, for example, 100 ml HCl with unknown concentration. In addition, there is an indicator that produces a color change at a neutral pH value.
- Addition of the measuring solution: With stirring and/or shaking, a measuring solution is dribbled into the sample solution in the next step. This contains the titrator at a known concentration, such as NaOH at 0.1 mol/l.
- Reaching the equivalence point: The two solutions now react with each other, in which case the following equation applies:
| NaOH + HCl ? NaCl + H2O |
With the help of the burette, the measuring solution is dripped into the sample solution until the equivalence point is reached. This point is similar to the neutral point for strong acids and bases, so that there is a pH of 7. This is indicated visually by the color change of the indicator. In the solution is now exactly the same amount of hydrochloric acid and sodium hydroxide, so
| n(NaOH) = n(HCl) |
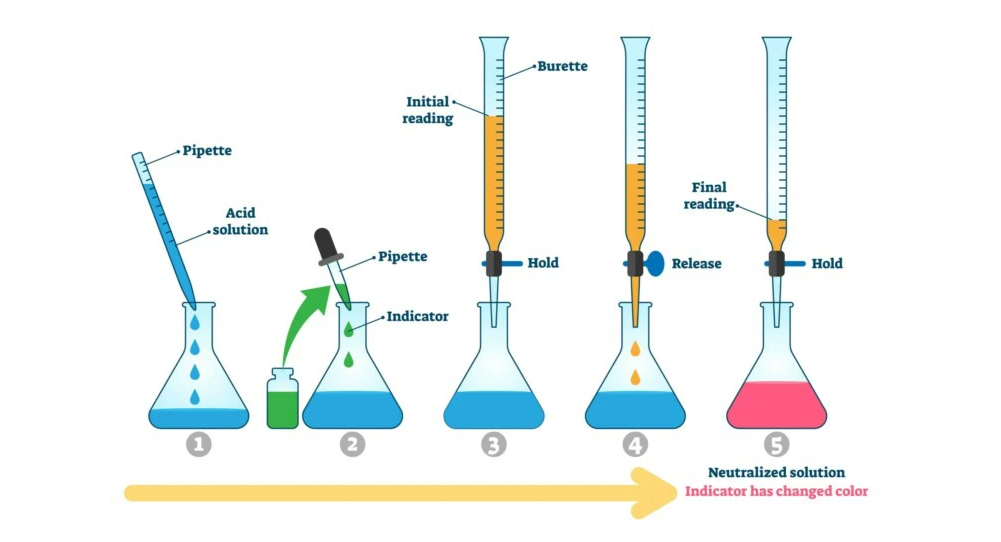
Titration: Aufbau des Versuchs
Damit ein exaktes Messergebnis erzeugt werden kann, sollte beim Versuchsaufbau auf einige Dinge geachtet werden. Dazu gehören neben genauen Abmessungen auch das richtige Equipment und die optimale Vorgehensweise.
1. Titration: Geräte für das Verfahren
Um eine Säure-Basen-Titration durchzuführen, werden für den Titrations-Versuchsaufbau üblicherweise erst einmal die folgenden Geräte benötigt:
- Bürette
- Becherglas
- Stativ
- pH-Sensor oder Indikator
- Magnetrührer und Rührfisch
2. Chemikalien/Titrierlösung
Hinzu kommen unterschiedliche Stoffe. Bei einer Säure-Base-Titration werden verwendet:
- entweder eine starke Säure und eine starke Base
- oder eine Kombination aus starker Säure und konjugierter Base konjugierter Säure und starker Base.
Üblich ist allerdings die Kombination von Salzsäure und Natronlauge, wobei einer der Stoffe über eine bekannte Konzentration verfügt – das muss beim Dosieren der Fluide berücksichtigt werden. In our example, we will assume that the concentration of the caustic soda is 0.1 mol/l and that of the hydrochloric acid is unknown.
Often, a indicator is also added to indicate that the equivalence point has been reached with the aid of a color change. However, other methods may be used depending on the endpoint determination.
3. Titration: setting up equipment
The first step is to attach the burette to the stand so that it is above the beaker containing the unknown amount of hydrochloric acid. This in turn is placed on the magnetic stirrer. The known amount of sodium hydroxide solution is poured into the burette. It is important to work absolutely free of bubbles so that the amount consumed can be read off exactly later.
4. Insert the stirring fish
After that, the stirring fish can already be placed in the beaker. By turning on the magnetic stirrer, it will start to stir slowly. This can ensure that the two substances in the titration experiment setup are optimally mixed with each other – thus measurement errors can be easily avoided.
5. Immerse the electrode
Subsequently, the pH electrode can also be immersed. This is connected to a PC and continuously determines the pH value. If the equivalence point is reached at a pH of 7, the experiment can thus be terminated directly at the correct point.
Alternatively, an indicator can be used. This provides a color change when the equivalence point is reached, which also indicates a neutral pH. Consequently, a indicator solution must be selected whose color change is exactly at the correct point.
6. Start measurement
The measurement is started by slowly dropping the measuring solution with the burette into the beaker containing the sample solution. At a pH of 7, the measurement must be stopped in order to perform a successful titration and determine the substance concentration of the hydrochloric acid. There is the neutral point at which the two substances in the beaker have the same concentration.
7. Titration evaluation
As briefly explained under “Principles of titration setup”, the neutralization equation applies to our example:
| NaOH + HCl ? NaCl + H2O |
The consumed volume of NaOH (with a substance concentration of 0.1 mol/l) up to the equivalence point is also known by the slow dripping. This can be traced by a titration sketch.
This allows the titration to be calculated: for example, if the equivalence point is 0.05 l (to 100 ml of hydrochloric acid), the values can be inserted into the titration formula accordingly.
The following applies:
| n(HCl)/n(NaOH) =1 |
Conclusion:
| n(NaOH)=c(NaOH)*V(NaOH)=0,1 mol/L*0,05 L=0,005 mol=n(HCl) |
This gives 0.005mol/0.1L=0.05 mol/L as the concentration of hydrochloric acid.
Types of reactions in the titration setup
The type of reaction used to determine the concentration of a particular substance is not always the same – depending on the type of substance and indicator, several reaction types may be considered. These are distinguished by chemical reaction. Acid-base titration, for example, is particularly well known, but other variants are also possible.
Acid-base titration
Acid-base titration is used to determine the substance concentration of an acid or base with its counterpart. For this purpose, an indicator is usually used that changes color when the pH value of 7 is reached. In principle, this type of reaction can be differentiated into two subgroups
- Alkalimetry: In alkalimetry, the amount of acid is calculated by adding a known amount of substance of the base to the unknown amount of substance of the acid.
- Acidimetry: It runs the other way around in acidimetry or azidimetry. Here, a base amount is calculated by adding a known amount of acid to an unknown amount of base.
Precipitation titration
Precipitation titration does not involve a change in pH, but rather a precipitation reaction when the equivalence point is reached. This is visible by a precipitate in the solution, which depends on the substances used. Common precipitation reactions include, for example, the use of silver ions and chloride ions according to Gay Lussac and Liebig: this leads to a milky white precipitate.
In some cases, the reaction is aided by the addition of dyes to make the precipitate more visually recognizable. For example, in the Fajans titration, eosin or fluorescein are used. Titration according to Volhard, on the other hand, produces colored iron rhodanite (today it is more common to say iron thiocyanate) and according to Mohr silver chromate.
Redox titration
If there is talk of a redox titration, there is a complete conversion of the sample solution by the measured solution. The content of substances in the solution that can be oxidized or reduced is determined. In most cases, an intrinsic indicator is used to show the reaction end point – that is, the time at which the sample solution is completely reduced or oxidized.
Well-known redox titration methods are primarily manganometry, iodometry, bromometry and cerimetry. They are each named after the dimensional solution used.
Complexometric titration
Complexometric titration involves a complexation reaction, so again the name says it all. This type of reaction is used to determine the content of metal ions in a solution. The complexes bind to the metal ions, causing the solution to change color. Alternatively, the end of the reaction can be determined photometrically.
However, it is sometimes difficult to identify to which metal ion the complexes in the solution actually bind. Therefore, this method is considered less selective, which is why it is not suitable for every application.
Polyelectrolyte titration
It gets even more specific with polyelectrolyte titration. Here, the cationic demand for polyelectrolytes is determined, which corresponds to the charge intensity. The primary titrants used in this type of reaction are polyDADMAC (for an anionic suspension) or potassium polyvinyl sulfate (for a cationic suspension).
Phase transfer
In addition, the so-called phase transfer can be mentioned. It is used for determination of ionic surfactants present in an aqueous solution. A color change is also used as the endpoint in this method – namely that of the substance mixture in the organic-chlorinated phase.
Endpoint determination in titration setup
Speaking of endpoints, not only the reaction types can differ, but also the methods used to determine the endpoint. In titration performance, chemical and physical indicators can be distinguished in principle. Thus, the indicator solution allows visual (chemical) endpoint detection, while both potentiometry and conductometry proceed instrumentally (physically).
Indicator solution
Among the most common procedures is the addition of an indicator solution. This reacts to the pH in the solution and changes to a different color at a certain point. In an acid-base titration, the endpoint is at pH 7, so methyl orange or bromothymol blue are often used here. These have an envelope in the same latitude lines.
If, on the other hand, weak acids are titrated, the transition range must be correspondingly more basic. Methyl orange or bromothymol blue would turn over before reaching the equivalence point, which is why phenolphthalein is used instead in such cases.
Potentiometry
In potentiometry, on the other hand, the equivalence point is determined by a change in voltage. For this purpose, a measuring electrode (electrode of the 1st type) and a reference electrode (electrode of the 2nd type) are located in the sample solution and are connected to each other via a measuring circuit. While the measuring electrode is concentration-dependent, i.e. it depends on the sample solution, the reference electrode is concentration-independent.
The titration causes a change in concentration in the solution, so that a differential electrical potential is created between the electrodes. Finally, during neutralization, the end point can be measured at which the voltage changes abruptly. It can be mentioned as a great advantage of potentiometry that the measurement result is much more accurate than when using an indicator.
Conductometry
Conductometry can be considered as a third endpoint determination of the equivalence point. Here, the conductivity of the sample solution is measured continuously during the titration procedure. This works with the aid of an anode and a cathode as well as alternating current in the sample solution.
The more charge carriers present, the higher the conductivity. If the measured solution and the sample solution neutralize with each other, uncharged water is formed. This can no longer act as a charge carrier, so that the conductivity decreases. When the end point is reached, it increases sharply again because more new ions are added that can no longer be neutralized by the previously present sample solution.
Titration setup as the basis for correct measurements
Correct titration requires a tideless setup – only then is it possible to determine the concentration of a substance amount exactly. Various reaction types as well as diverse endpoint determinations are possible for this purpose. In any case, it is important that the methodology is adapted to the substance in question.
FAQ
For a successful titration, in addition to a measurement solution and the sample solution, a measuring device or an indicator is also required. Then the solutions are mixed by dripping until the equivalence point is reached.
The decolorization is generated by an indicator and represents a color change. This indicates that the end point has been reached, so that the titration procedure can be terminated. The concentration can then be calculated quite simply from the amount of substance consumed.
In the case of an acid-base titration, the titration curve shows both the amount of substance consumed and the associated pH value as well as the equivalence point . In addition, the initial pH value as well as the possible buffer range and pKS value can be read.
For a titration, such an indicator should always be selected whose color change is in the range of the end point . For strong acids and strong bases, for example, methyl orange and bromothymol blue are suitable. For weak acids, on the other hand, phenolphthalein is much more suitable.
Sie haben Fragen zum Thema oder möchten einen Themenvorschlag äußern? Kontaktieren Sie uns gern telefonisch unter +49 30 2096579 00 oder schicken Sie uns eine Mail an info@medsolut.com.




